Yesterday I was discussing the trending stories about the coming ice age (e.g., here) with my friend, Deborah Sloan.* She mentioned an unpublished paper she had written about the insignificance, with respect to inducing changes in Earth’s temperature, of the “greenhouse effect.” Her paper, published below, is somewhat technical. But it’s worth the time and effort, as it will help you to evaluate the claims being made on both sides of the debate (today The Washington Post published what purports to be a rebuttal).
Bottom line: The current concentration of CO2 in our atmosphere is already absorbing nearly all the heat capable of being absorbed in this manner, so that further CO2 increases can have little effect. Significant temperature increases can be achieved only by adding significantly more energy from the sun. Accordingly, if we lose energy from the sun, as the new models (importantly not yet published for scrutiny) predict, additional CO2 can do little or nothing to make up for the difference.
The greenhouse effect is commonly presented as a malevolent, man-made mechanism leading to catastrophic anthropogenic global warming. In this context, we are being called upon to make fundamental changes to our way of life by abandoning CO2-producing fossil fuels. Must we? Is this really true? Given the stakes — the dramatic changes that may be forced upon us by means of government action — this is an important thing to understand.
The greenhouse effect is an increase in average temperature at and near the surface of a planet, which results from the interaction of light with gasses in that planet’s atmosphere. The first essential thing to grasp here is that the warming results from the interaction of light with matter.
Interaction of light with matter
Light is not heat. Heat is the kinetic energy of molecular motion, whereas light is an electromagnetic phenomenon; a disturbance propagating through electromagnetic fields, like ripples in a pond. The various types of light (e.g. infrared, microwave, ultraviolet, visible) are distinguished by their characteristic ranges of wavelengths, just as green and yellow light are distinguished within the visible range.
The types of interactions of light with matter include transmission (like a window pane), reflection (like a mirror) or absorption it (like a strip of asphalt on a sunny day).
Energy from the sun
The sun radiates energy in the form of light with a peak intensity in the visible range of wavelengths. In the presence of visible light, atmospheric gases act like a window, allowing most of the light to pass through. However the surface of the earth acts mostly like asphalt, absorbing a significant fraction of the light and converting it into heat.
In the process of absorption, a photon of light interacts with the electromagnetic attributes of a molecule, and thereby causes an increase in the internal energy of the molecule (be it a change in the orbital configuration of an electron cloud to a higher energy state or a spring-like vibration of the molecule’s constituent atoms).
The net result of energy absorption at the earth’s surface is that the molecules get excited and the surface gets warmer.
Equilibrium
The earth does not just keep getting arbitrarily hot over time as it continues to absorb energy from the sun. Rather, it reaches a state of equilibrium — a global average temperature at which the rate of energy loss is equal to the rate of energy absorbed. Earth’s mechanism for losing energy is a process called radiative cooling. Radiative cooling entails the emission of energy in the form of photons (light), which radiate out into space. Unlike the sun, the earth emits energy in the form of infrared light as opposed to visible light. This difference will become important later in our discussion.
The process of emitting photons can be thought of as the inverse of the absorption process: a molecule falls to a lower internal energy state as it emits energy in the form of a photon. Ultimately this results in a decrease in molecular kinetic energy, and the aggregate effect is cooling.
Radiative cooling is the earth’s only possible means of shedding energy back out into space. That would be true regardless of the composition of the atmosphere, and it would be true even if we had no atmosphere at all.
Effect of the atmosphere
If there were no atmosphere, however, the earth would be much cooler. In the presence of an atmosphere, molecules that comprise the materials of earth’s surface, which have absorbed energy from the sun, can transfer some of that energy to atmospheric gases by means of molecular collisions.
Imagine a baseball pitched to a brick wall: neglecting the effects of drag it would simply bounce back without any change in kinetic energy. Now imagine we replace the brick wall with Barry Bonds, who cracks the ball with his bat and sends it soaring out of the field with an increased kinetic energy.
The latter scenario is essentially what happens when atmospheric gas molecules, bouncing around as they do, collide with higher energy molecules. The high energy molecules act like Barry Bonds, and impart the gas molecules with increased kinetic energy.
The result of this interaction is that, rather than being lost via radiative cooling, some of the earth’s absorbed energy is converted into molecular kinetic energy, i.e. heat, in the atmosphere (recall that heat is the energy of molecular motion). The resulting equilibrium temperature is higher than what it would be without an atmosphere.
This, however, is not the greenhouse effect and would occur even without any greenhouse gases.
The greenhouse effect
Though warmer than it would be in the absence of an atmosphere, the earth would still be very cold if it had no greenhouse gases: the average temperature near the surface would be about -18°C.
Oxygen and nitrogen can capture some of the absorbed energy by interacting with the surface materials. Nonetheless, as we have seen, a significant amount of energy still escapes past this “initial line of defense” in the form of emitted infrared radiation. Without an additional warming mechanism the earth would still cool itself too effectively, with an equilibrium temperature below that at which water freezes.
Fortunately, some atmospheric gases act like asphalt in the presence of infrared light: these gases can absorb the infrared light that is emitted from the earth’s surface, rather than transmitting it the way that oxygen and nitrogen do. These are the greenhouse gases (GHGs) such as carbon dioxide (CO2).
Returning to our baseball analogy, the energized GHG molecules interact with the other molecules as Barry Bonds would do with a baseball. When an excited CO2 molecule absorbs an IR photon and then collides with an oxygen molecule, it may transfer some of its internal energy to that oxygen molecule, which then has an increased kinetic energy after the collision. The aggregate effect is heating; an increase in the earth’s equilibrium temperature as compared to what it would be without GHGs. That is the greenhouse effect.
An interim summary
Before we reach a final and critical point, the interrelated mechanisms discussed thus far can be summarized as follows:
- The sun emits energy in the form of mostly visible photons
- Some of that energy reaches and is absorbed by earth’s surface
- Some of the absorbed energy is dispersed into the atmosphere as a result of gases interacting with the surface
- Some of the absorbed energy is re-radiated from the surface in the form of mostly infrared photons
- Some of the re-radiated energy is absorbed again in the atmosphere by GHGs and subsequently dispersed into the atmosphere by molecular collisions
One might validly ask at this point: what if there is too much heating, and that becomes harmful to us? The answer is, that can’t happen. The reason is saturation.
Infrared saturation and diminishing returns
The amount of energy absorbed and re-emitted by the earth is finite. The re-emitted energy is distributed over a range of wavelengths of light in the infrared band. At certain critical wavelengths within this band, — mostly those at which CO2 or water can absorb photons — the band is saturated.
As fast as earth’s surface can emit photons at the saturated wavelengths, the GHGs in the atmosphere absorb them and disperse that energy into the atmosphere. All of the energy emitted at those wavelengths is already intercepted and converted into heat, so no further warming effect is possible at those wavelengths — not even if the atmosphere were comprised of pure CO2.
There are other wavelengths at which CO2, water and other GHGs absorb IR light in a much weaker manner, kind of like a dirty window pane as opposed to a slab of asphalt. These wavelengths are not yet saturated, which means that the absorption in these ranges of wavelengths can still go up with increased amounts of CO2 in the atmosphere. However this increase will occur with an exponentially diminishing return.
This is to say, the greenhouse effect is logarithmic. The following graph, created via absorption/emission simulations, illustrates the logarithmic variation of temperature increase with CO2 concentration (see also here), in a simplified scenario with a uniform temperature distribution in the atmosphere, a constant atmospheric thickness and no other GHGs besides CO2:
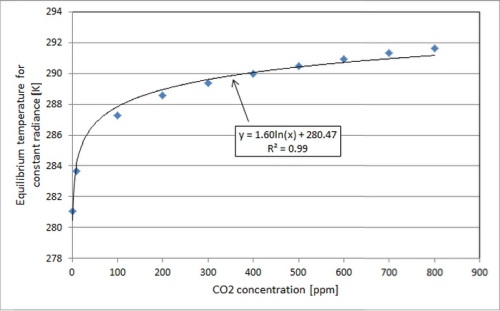
An important thing to note here is not the exact numerical values for temperature as a function of CO2 concentration, but the trend. Our current CO2 concentration is about 400 ppm, so you can see that most of its warming potential has already been realized — the curve is pretty flat as we move up to higher concentrations of CO2.
Another important thing to note is that despite its mildness, the temperature variation trend indicated above is more severe than what we could expect to see in a real-world scenario. This is because CO2 is not the only major GHG in earth’s atmosphere: for instance, water is a huge IR absorber and there is much more of it in the atmosphere than there is CO2, contributing to a greater present degree of saturation. Because this is the case, the increase in global average temperature per unit of CO2 increase would actually be smaller than the graph above suggests.
Conclusions
Science is often wielded before the public as a means of intimidation: dire predictions are made in the name of science and, due to the division of labor inherent in an advanced civilization, most people lack the specialized knowledge required to deal with those claims. My purpose here has been to combat that intimidation by explaining and de-mystifying the basic mechanisms associated with the greenhouse effect. There is no rational basis for concluding that increased atmospheric CO2, as a by-product of an abundant, life-preserving industrial civilization, are a threat to mankind.
*Deborah Sloan is a mechanical engineer and a researcher at the Center for Industrial Progress. Learn more about industrial progress on Facebook.
See (actually, hear) also: Alex Epstein, Founder and President of Center for Industrial Progress, interviews Dr. Craig Idso about the benefits of CO2


You state, “The sun emits energy in the form of mostly visible photons.”
I do not think that is true. Visible light is only a small part of the spectrum, and higher frequencies equate to higher energies.
Using data from the Vostok Ice Cores, CO2 concentrations varied from about 200-300 ppm while temperature varied by about 10 degrees C. Your chart shows an equilibrium change of only about 2 degrees C for the same change in CO2. Was it presumably out of equilibrium, different gas concentrations, does the fit just not work for the whole earth, or what?
Pingback: “Ice Age, Nuclear Winter or Frozen Abstractions, Which Will Ice You First?” Tonight at 11 p.m. ET (8 p.m. PT) | Don't Let It Go
This data has been known and accepted science by the IPCC, as I recollect. It is crucially important, but hasn’t been disseminated. Broadly speaking, there are only two ways that CO2 can increase the temperature of the earth. First, its own greenhouse gas effect; secondly, it can cause other effects by influencing processes that heat the earth. So, we see that there is a saturation of CO2 ‘s ability to increase temperature, and that the current concentration is at the flattened portion of the curve. This method at even the most aggressive increases in CO2 will not be able to increase the earth’s temperature more than about 2°Kelvin over the next hundred years. Not a worry as that might get us back to the Roman warm period or the Medieval warm period.
Now, what about the second method of warming? That is, in which CO2 causes other processes, sometimes called forcings, to force the earth’s temperature to rise. The Carbon Climate Creationists would have us believe that every possible forcing would be engaged and that there is ample historical evidence of this occurrence. Actually, there is none. The empirical data come from the IPCC, believe it or not. It is known that CO2 has risen and fallen throughout earth’s history. In fact, CO2 concentrations have been 15X the current level without any evidence that secondary forcings have taken place. Indeed, the CO2 concentration has been higher during an ice age.
I personally wish CO2 could warm the planet, as humanity has flourished during warm eras. For this we have historical accounts. Additionally, Craig Idso has studied and demonstrated the enormous benefit of increasing carbon dioxide upon plant life, and therefore all life. The computer model fantasies of the Carbon Climate Creationists have exactly NO history or empirical data to support their position. It continues to astonish me that this warming hoax is perpetrated on a gullible public. In my region the coal industry is limping into the grave despite generations of coal still waiting to be unearthed to provide power to humanity.
(Note to Phil – yes, at different -lower- CO2 concentrations, there is a greater ability to hold heat. That is the reason for the logarithmic curve. Also, the ice core data doesn’t tease out the enormous contributions from water vapor, cloud cover, the orbital mechanics of the earth around the sun, etc. As I said, these had huge effects on the temperature and were wholly unrelated to CO2 concentration.)
“This method at even the most aggressive increases in CO2 will not be able to increase the earth’s temperature more than about 2°Kelvin over the next hundred years. Not a worry as that might get us back to the Roman warm period or the Medieval warm period.”
Wikipedia would contradict you:
https://en.wikipedia.org/wiki/Temperature_record
The average temperature of the Earth in the 2010s is the highest it has ever been for the past 12,000 years, since the end of the last ice age. In fact, you would have to go back more than 100,000 years to have an average Earth temperature that is higher than what exists now.
Also, an increase in temperature of about 2 Kelvin would place us at very close to, or over, the maximum average temperature of the Earth for the past eight-hundred thousand years!! All this information is easily available in the temperature graphs at the link above.
Lastly, Carbon-dioxide emissions, by themselves, if remained unchecked, are enough to send us more than 2 Kelvin upward from where we are now. Meanwhile, methane and nitrous-Oxide are between 34 and 300 times more potent as greenhouse gases than Carbon-dioxide is. Both the coal-mining and the meat industries are responsible for emitting vast amounts of methane gas into the atmosphere, and we haven’t even factored in their effects yet. I think there is plenty of reason to take precautions before dismissing the research and policy of the overwhelming majority of working climate scientists.
I would not rely on Wikipedia for your temperature data. Quite simply, and frankly, that is not true. Peer-reviewed science is the temperature data I was quoting. A sample of this can be found at Quaternary Science Review 19, 213-226 (2000). This is from Greenland Ice Core data, but similar data is found from all over the world.
It is true, I was optimistic when hoping for a return to the Holocene Climactic Optimum, about 7000-9000 years ago, most of which was 1.5K, not 2K higher than now. I admit to being a bit hopeful about increasing the temperature back to the warm periods of historical mankind. I stand corrected – 1.5K. In point of fact, your statement that the current warm period is the warmest since the last ice age is not true. The Medieval warm period was warmer than today. The hype about current temperature is simply laughable.
Most worrisome, though, is the notion that higher temperature and increasing CO2 would be anything but beneficial for all life on earth. I only WISH it were true that CO2 could increase temperature as much as the Carbon Climate Creationists state it will. We are carbon-based life forms, as is all life on earth. CO2 is a normal, non-pollutant constituent of the carbon cycle, necessary for all life on earth. In fact, if CO2 got below about 150 ppm, photosynthesis would cease. So, please, for the sake of life on earth, please mine more coal, drill more oil, and fracture more shale. It will green the earth.
One need go only back about 1000 years to see higher global mean temperatures than today, not 12,000 as is stated in the prior post. There are contemporaneous historical accounts for this as well. Who, in his right mind, would name that land mass Greenland? And yet it was so named, and there is evidence of grape cultivation in Greenland during the Medieval warm period. That could NOT be done today, because it is much cooler now than it was then. While it is true that we are in the midst of a cyclic warm period when studied since the Holocene Climactic Optimum, there is a good deal of evidence from solar activity that we may be heading for a cool period, much closer to the Little Ice Age that was present during the American colonial period. I hope for my sake and that of my children’s, that the cycle of warmth that appeared in the late 1900s will return. The trend of temperature since the Holocene Optimum is clearly cooling, however.
CH4 and N20. Wow, the Climate Creationists are stretching now. During college I actually worked in West Virgina’s coal mines measuring methane at the face of the coal seam. It is criminal that our EPA is closing down the most abundant and inexpensive source of energy that we have. All the while sticking it to the UMWA, their political brethren. Some people will never learn.
Oh, and with respect to much of the recent temperature data: I wouldn’t trust many due to proven political manipulation, but especially the Carbon Climate Creationists. They have already been found colluding to outright change both current and historic data to fit their doomsday theories (East Anglia emails revealed). It is a shame the mass media has bought this hook, line, and sinker: the recent few weeks of warm weather has been looked at frantically by Al Roker. This is the exact same warm weather that when I was a kid we called “SUMMER.”